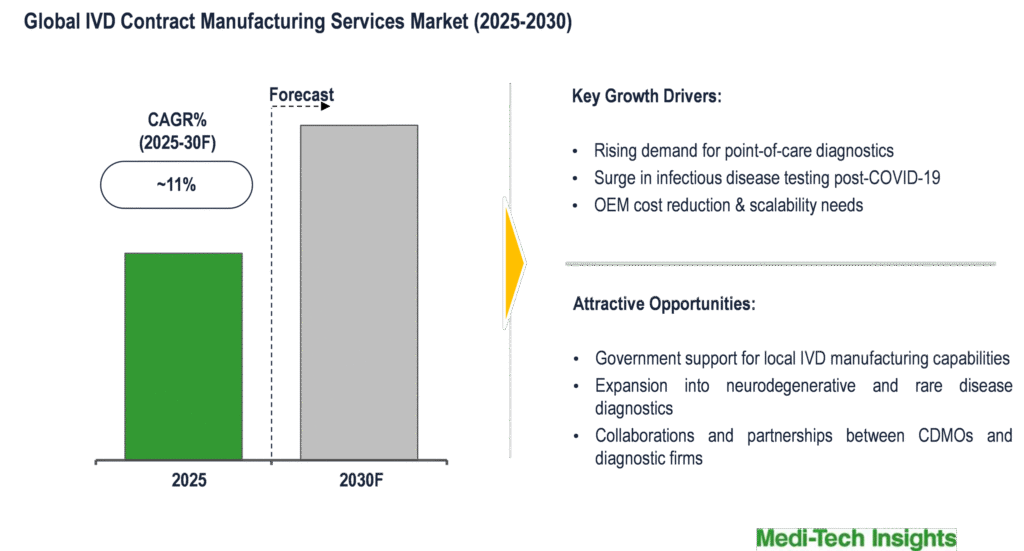
The global IVD contract manufacturing services market is set to witness a growth rate of ~11% during the forecast period. Growing preference for point-of-care testing services; government support to scale up production of IVD kits post COVID-19 pandemic; growing incidence of cancer and infectious diseases; and advantages such as cost savings, scalability, technology know-how and risk mitigation offered by IVD contract manufacturers are some of the key factors driving the market growth.
In-vitro diagnostics (IVD) are tests done on samples such as blood or tissue to detect diseases or other conditions. It is used to monitor a person’s overall health to help cure, treat, or prevent diseases. Cost savings, scalability, technology know-how, and risk mitigation offered by IVD contract manufacturers drive OEMs’ decision to outsource to IVD CDMOs.
Download a free sample report now 👉
https://meditechinsights.com/ivd-contract-manufacturing-services-market/request-sample/
Growing preference for point-of-care (POC) testing services fuels the growth of the IVD contract manufacturing services market
In an endeavor to cut result time and help patients make better-informed decisions about their health, there is considerable demand for IVD testing to move closer to the patient, i.e. point-of-care (POC) diagnostics, whether in the hospital, clinic, physician’s office, or home. Manufacturing of specific PoC devices presents manufacturers (OEMs) with a variety of design and manufacturing challenges. For instance, the ability to process high-precision components that involve reagents and fluids are the most relevant capabilities sought by OEMs, which are accomplished by specialized IVD CDMOs.
“Rising demand for point-of-care IVD devices is a key driver for growth. Recently launched point-of-care IVD instruments and devices are convenient to use and efficient. Manufacturing of specific PoC devices presents manufacturers (OEMs) with a variety of design and manufacturing challenges, which is addressed by IVD CDMOs.” – Director, Leading IVD OEM, United States
Cost savings, scalability, technology know-how and risk mitigation offered by IVD contract manufacturers drives their demand
By partnering with IVD contract manufacturers, OEMs can save costs on labor, automation and can achieve economies of scale at a relatively lower cost. OEMs prefer IVD CDMOs for their scalability, reliability, and cost-effective manufacturing capabilities. OEMs look for IVD CDMOs who have sound technical capabilities that are not part of their core competency. Mitigation of regulatory risks & supply disruption risks is other pivotal factors that urge OEMs to partner with IVD CDMOs. For instance,
- In January 2025, Akoya Biosciences selected Argonaut Manufacturing Services as its contract manufacturing partner to support the production of in-vitro diagnostic (IVD) products, aiming to accelerate commercialization and expand its IVD offerings
- In March 2024, Beckman Coulter and Fujirebio collaborated to co-develop patient-friendly, blood-based Alzheimer’s disease tests, leveraging Fujirebio’s IVD expertise and manufacturing capabilities
Growing incidence of cancer and infectious diseases & role of IVDs drives the IVD contract manufacturing services market
IVD plays a critical role in driving clinical decision-making for cancer screening, diagnosis, and treatment. IVD testing provides insights related to a patient’s health status such as risk or predisposition for developing certain cancer; the stage of disease, and the prognosis for progression/remission after therapy.
IVDs are also used to analyze human samples such as blood and saliva. It helps in measuring the concentration of specific substances/analytes, detects the presence or absence of a particular marker or set of markers in response to infection. Clinicians frequently use IVDs to diagnose infectious diseases, guide treatment decisions and even mitigate or prevent future diseases. Continuously rising cases of cancer and infectious diseases are likely to spur the IVD contract manufacturing services market.
Competitive Landscape Analysis
The IVD Contract Manufacturing Services market is marked by the presence of key players such as Jabil Inc., STRATEC, Celestica, Sanmina Corporation, Röchling, KMC Systems, Savyon Diagnostics, Nova Biomedical, Cone Bioproducts, Thermo Fisher Scientific Inc., Cenogenics Corporation, Coris BioConcept, Gerresheimer, and Phillips-Medisize, among others. Some of the key strategies adopted by market players include strategic partnerships and collaborations and geographic expansion.
Download a sample report for in-depth competitive insights
https://meditechinsights.com/ivd-contract-manufacturing-services-market/request-sample/
Global IVD Contract Manufacturing Services Market Segmentation
This report by Medi-Tech Insights provides the size of the global IVD contract manufacturing services market at the regional- and country-level from 2023 to 2030. The report further segments the market based on service, technology, and end-user.
Market Size & Forecast (2023-2030), By Service, USD Million
- Manufacturing Services
- Assay Development Services
- Others
Market Size & Forecast (2023-2030), By Technology, USD Million
- Immunoassays
- Clinical Chemistry
- Molecular Diagnostics
- Hematology
- Microbiology
- Coagulation
- Others
Market Size & Forecast (2023-2030), By End-user, USD Million
- Medical Device Companies
- Academic & Research Institutes
- Others
Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
info@meditechinsights.com