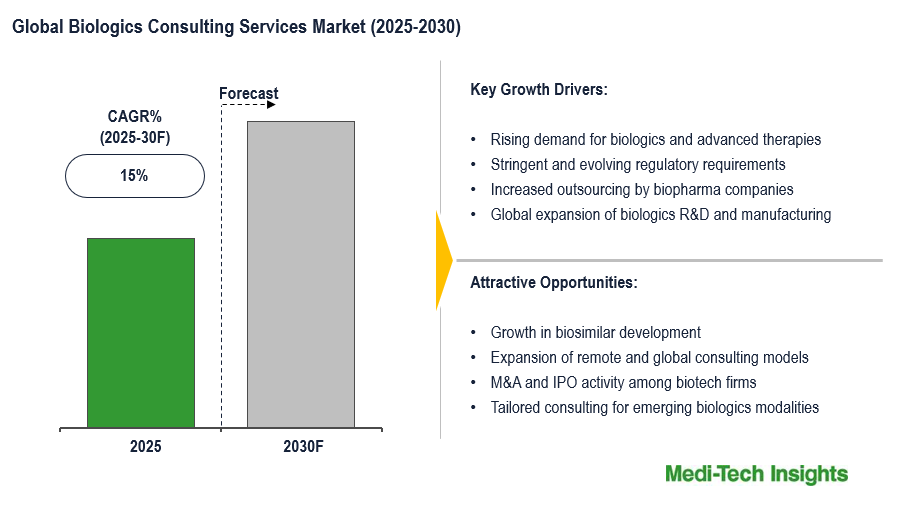
The global biologics consulting services market is set to witness a CAGR of ~15% in the next 5 years. Rising demand for biologics and advanced therapies, stringent and evolving regulatory requirements, increased outsourcing by biopharma companies, and global expansion of biologics R&D and manufacturing are some of the key factors driving the biologics consulting services market.
Biologics consulting services offer specialized advisory to pharmaceutical and biotechnology companies throughout the development, regulatory, and commercialization lifecycle of biologic products such as monoclonal antibodies, vaccines, biosimilars, cell and gene therapies, and advanced biologics. Biologics consulting services not only assist clients through complicated regulatory pathways (e.g., FDA, EMA), optimize clinical trial strategies, ensure Good Manufacturing Practice (GMP) compliance, and address Chemistry, Manufacturing and Controls (CMC) needs, but make use of consultants who are often former regulatory agency employees, scientists, or industry professionals. Thus, as biologics are more and more incorporated into healthcare, biologics consulting services help accelerate the product development path, reduce potential risks, and ensure successful market entry in a global marketplace.
To request a free sample copy of this report, please visit below
https://meditechinsights.com/biologics-consulting-services-market/request-sample/
Rising demand for biologics and advanced therapies to drive market growth
The growing demand for biologics and advanced therapies (such as monoclonal antibodies, gene therapies, cell therapies and mRNA therapies) is creating significant development in the biologics consulting space. As these therapies continue to be used more frequently to treat cancers, autoimmune diseases, and rare genetic disorders, companies’ development, regulatory compliance and manufacturing processes are increasingly becoming complex. This added complexity drives companies to seek consulting expertise for strategy, regulatory submissions, and clinical planning, which is unique to biologics. Consulting is also critical because most biopharma companies do not have in-house biologics development expertise. Consulting services can lead to faster timelines, reduced risk and potentially successful development and commercialization of biologics and advanced therapies.
Increased outsourcing by biopharma companies to boost market growth
Most biotech startups or mid-sized pharmaceutical companies are unable to hire the personnel to build a complete team with in-house expertise to manage the complex processes for biologics development and commercialization. As a result, biopharma companies turn to specialized consulting firms for advisory assistance in various areas of the biologics development process, such as regulatory strategy, clinical trial design, manufacturing compliance and quality assurance, etc. Outsourcing allows biopharma companies to have asset-lean operations, reducing overall costs, but being able to tap into summarized experience and expertise as needed. There is an increasing outsourcing trend because new biologic pipelines continue to expand. Therefore, there is consistent demand for consulting services outright as a part of any biologics development lifecycle activity that requires consulting.
Competitive Landscape Analysis
The global biologics consulting services market is marked by the presence of established and emerging market players such as IQVIA; ICON plc; Syneos Health; Biologics Consulting Group, Inc.; Parexel International (MA) Corporation; Lachman Consultant Services, Inc.; PHARMALEX GMBH (Cencora); Validant; Regulatory Compliance Associates Inc; and DSI InPharmatics; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
🔗 Want deeper insights? Download the sample report here:
https://meditechinsights.com/biologics-consulting-services-market/request-sample/
Global Biologics Consulting Services Market Segmentation
This report by Medi-Tech Insights provides the size of the global biologics consulting services market at the regional- and country-level from 2023 to 2030. The report further segments the market based on phase, product type, and end user.
Market Size & Forecast (2023-2030), By Phase, USD Million
- Preclinical
- Clinical
- Commercial
Market Size & Forecast (2023-2030), By Product Type, USD Million
- Monoclonal Antibodies (mABs)
- Vaccines
- Cell Therapies and Gene Therapies
- Biosimilars
- Fusion Proteins and ADCs
- Oligonucleotide Therapies
- Others
Market Size & Forecast (2023-2030), By End User, USD Million
- Biopharmaceutical Companies
- Academic and Research Institutions
- Others
Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
info@meditechinsights.com