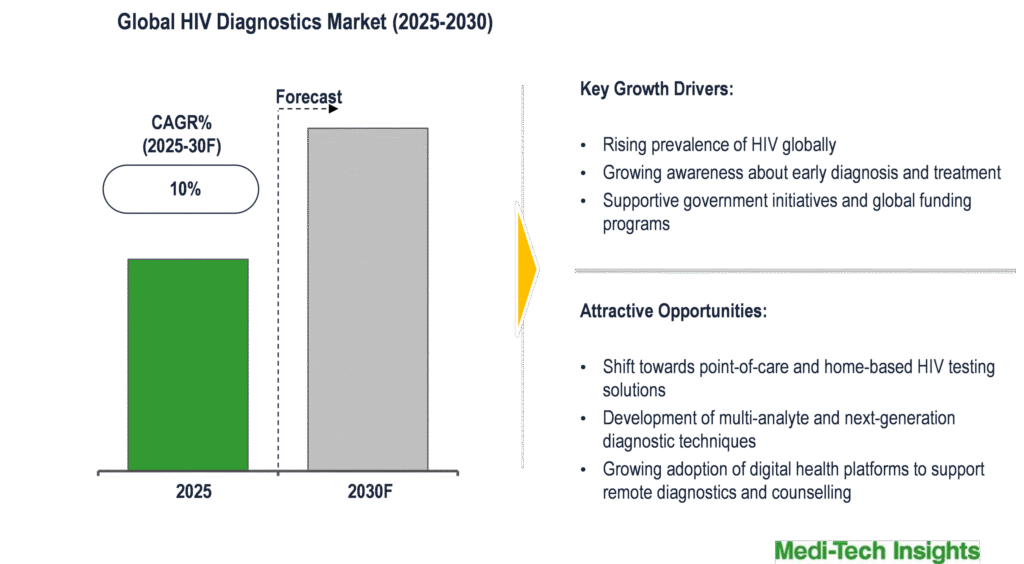
The global HIV diagnostics market is expected to grow at a CAGR of ~10% during the forecast period. Rising prevalence of HIV globally, supportive government initiatives, increasing awareness about early detection, and growing adoption of rapid self-test kits and point-of-care diagnostics are key factors driving the market growth. However, higher costs of advanced HIV diagnostic technologies, lack of proper infrastructure in low and middle-income regions, and social stigma remain significant barriers to the widespread adoption of HIV testing.
HIV diagnostics refer to the methods and procedures used to detect the presence of Human Immunodeficiency Virus (HIV). Accurate and timely diagnosis not only helps in appropriate medical treatment but also helps prevent further transmission within communities. Some of the HIV diagnostic tests include:
- Antibody Tests: These are the most common initial screening tools, which detect antibodies produced by the immune system in response to HIV infection
- Antigen/Antibody Combination Tests: Also known as 4th generation tests, these detect both HIV antibodies and the p24 antigen, which appears soon after infection. This combined detection enables earlier identification of HIV infections compared to tests that detect only antibodies
- Nucleic Acid Tests (NATs): These tests identify the actual genetic material (RNA) of the virus in the blood. NATs are highly sensitive and are used for early detection and to monitor viral load in infected individuals
- Rapid Diagnostic Tests (RDTs): These are point-of-care tests that deliver results within 20 to 30 minutes using blood or oral fluids. These tests are widely used in community settings and outreach programs due to their convenience and speed
- Self-Testing Kits: Designed for personal use; these kits allow individuals to collect samples and test themselves for HIV at home. They increase accessibility and reduce the stigma associated with clinical testing
These diagnostic methods play a crucial role in HIV prevention, treatment, and management by enabling healthcare professionals to promptly diagnose HIV infection, initiate appropriate interventions, and prevent further transmission of the virus.
Download a free sample report for in-depth market insights
https://meditechinsights.com/hiv-diagnostics-market/request-sample/
Government initiatives and global funding: expanding access to HIV diagnostics
Government initiatives and global funding programs aimed at diagnosing and reducing the rising incidences of HIV/AIDS are playing a key role in driving the HIV diagnostics market. Across the globe, public health organizations are partnering with governments to make HIV testing more accessible and affordable. These efforts are backed by government-funded programs which are strategically planned to improve public health outcomes. For instance, the US led PEPFAR (President’s Emergency Plan for AIDS Relief) is a landmark global initiative to increase HIV testing and has helped millions of people around the world to get tested for HIV. Similarly, the Global Fund provides financial and logistical support to increase the HIV testing programs, especially in regions like sub-Saharan Africa and Southeast Asia, where the rate of HIV infections is high. National governments are also stepping up. According to an article by UNAIDS, countries such as South Africa and Brazil have also made HIV testing a central part of public healthcare and increased access in rural and remote areas through mobile clinics and point-of-care diagnostic tools.
In addition to large-scale testing campaigns, governments are supporting innovation in HIV diagnostic techniques by speeding up regulatory approvals, funding for research and development. Overall, strong government support and public awareness programs are significantly improving access to HIV testing and helping reach more people around the world. For Instance,
- In December 2024, the Global Fund and PEPFAR announced a coordinated effort to provide lenacapavir, a long-acting injectable HIV pre-exposure prophylaxis (PrEP), to at least 2 million people over three years. This collaboration is supported by the Children’s Investment Fund Foundation and the Bill & Melinda Gates Foundation, aiming to reduce HIV infection rate
- In January 2024, the Global Fund approved US$9.2 billion in new grants to support HIV and other disease awareness and testing programs across more than 70 countries. This funding aims to strengthen health systems and expand access to essential diagnostics and treatments, significantly impacting the HIV diagnostics market
From clinics to comfort: the growing adoption of HIV self-test kits
In recent years, the shift towards self-testing of HIV at home has gained remarkable momentum. This change is mainly driven by technological advancements and a growing focus on privacy, convenience and accessibility for HIV testing. Lately, multiple innovations have taken place for developing more accurate and user-friendly HIV self-test kits. Companies have developed HIV test kits which use an oral swab to detect HIV antibodies and give results in approximately 20 minutes. These tests have demonstrated high levels of sensitivity and specificity, which makes them more reliable and has reduced the discomfort often associated with blood-based diagnostic methods.
Governments and health organizations around the world are increasingly including HIV self-testing in their public health strategies to improve early detection and reduce transmission of the virus. Self-testing kits allow individuals to test in the privacy of their homes, which also reduces the stigma often associated with HIV testing and is especially beneficial for people who feel anxious or uncomfortable about visiting healthcare centers for HIV diagnosis. The increasing adoption of HIV self-testing is expanding access to HIV testing, reducing social stigma, and supporting early detection and treatment efforts. For instance,
- In January 2025, OraSure Technologies received FDA approval for its OraQuick HIV Self-Test, to be used by individuals aged 14 and older, increasing access among adolescents in the US
- In June 2023, Newfoundland Diagnostics partnered with Atomo Diagnostics to sell HIV rapid self-test kits in retail outlets like Tesco across the UK/EU, marking the first commercial HIV self-test supply in mainstream European retail channels
- In April 2023, Abbott partnered with Population Services International (PSI) to offer HIV self-test kits at a fixed, affordable price in low- & middle‑income countries, thereby expanding access and reducing social stigma associated with clinic visits
Competitive Landscape Analysis
The global HIV diagnostics market is marked by the presence of established and emerging market players such as Abbott Laboratories, F. Hoffmann-La Roche Ltd., BioMérieux S.A., Siemens Healthineers, Danaher Corporation, OraSure Technologies, Chembio Diagnostics Inc., Becton, Dickinson and Company (BD), Thermo Fisher Scientific Inc., and Hologic, Inc., among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Unlock key data with a sample report for competitive analysis:
https://meditechinsights.com/hiv-diagnostics-market/request-sample/
Global HIV Diagnostics Market Segmentation
This report by Medi-Tech Insights provides the size of the global HIV diagnostics market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product, test type, and end user.
Market Size & Forecast (2023-2030), By Product, USD Million
-
- Reagents & Consumables
- Instruments/Devices
- Software & Services
Market Size & Forecast (2023-2030), By Test Type, USD Million
-
- Antibody Tests
- Antigen/Antibody Combination Tests
- Nucleic Acid Tests (NAT)
- Viral Load Testing
- Other
Market Size & Forecast (2023-2030), By End-user, USD Million
-
- Hospitals & Clinics
- Diagnostic Laboratories
- Home Care Setting
- Research Institutes
- Blood Banks
Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
info@meditechinsights.com
