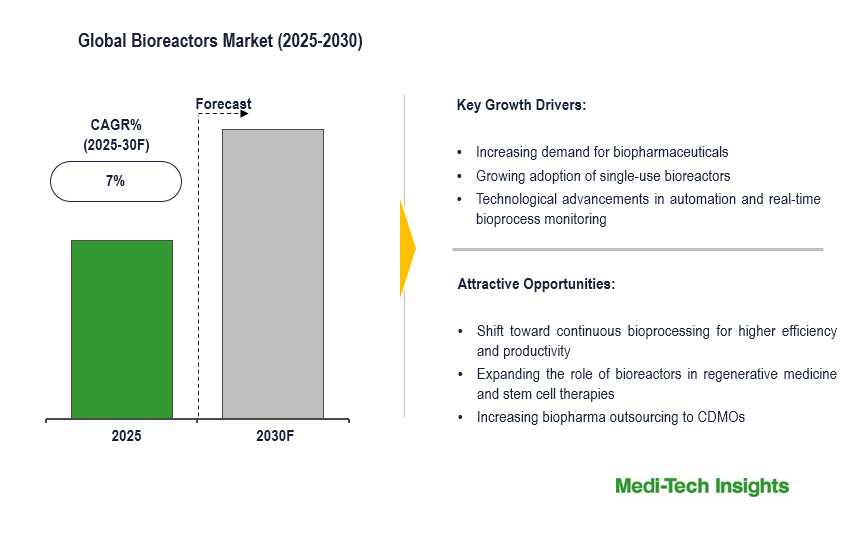
The global bioreactors market is expected to grow at a CAGR of ~7%, driven by increasing demand for biopharmaceuticals like monoclonal antibodies, gene therapies, and advanced vaccines. The adoption of single-use bioreactors (SUBs) is accelerating due to their advantages in reducing downtime & contamination risks and enhancing manufacturing flexibility. CDMOs are heavily investing in SUBs to support biologics production, particularly in personalized medicine and rare disease treatments. However, high capital costs, stringent regulatory approvals, and scalability challenges remain key hurdles for large-scale bioprocessing.
Bioreactors are controlled environments designed for biological reactions that enable the growth of cells, microorganisms, or tissues for applications in pharmaceuticals, biotechnology, and bioengineering. These systems play a crucial role in producing monoclonal antibodies, vaccines, and cell-based therapies, where precision, sterility, and scalability are essential. Traditional bioreactors, such as stirred tanks or airlift designs, have long been industry standards, but advancements in automation and single-use technologies are reshaping the landscape. The latest bioreactors integrate real-time monitoring, adaptive control mechanisms, and AI-driven process optimization to enhance biomanufacturing efficiency while maintaining stringent regulatory compliance.
To request a free sample copy of this report, please visit below
https://meditechinsights.com/bioreactors-market/request-sample/
Expanding role of biopharmaceuticals in market growth
The growing demand for biopharmaceuticals is the most significant force shaping the bioreactors market. Unlike traditional small-molecule drugs, biologics such as monoclonal antibodies, recombinant proteins, and cell-based therapies require highly controlled environments for production, making bioreactors indispensable. The increasing prevalence of chronic diseases, including cancer, autoimmune disorders, and rare genetic conditions, has driven pharmaceutical companies to expand bioprocessing capabilities, ensuring high-yield, high-purity biologics.
In addition to established therapies, cell and gene therapies (CGT) have emerged as novel therapies, with CAR-T cell treatments and regenerative medicine demanding highly specialized bioreactor systems. To meet this demand, companies are shifting from traditional stainless-steel bioreactors to single-use models, allowing faster turnaround between batches and reducing cleaning validation requirements. Additionally, partnerships between biopharma firms and CDMOs have surged, enabling more efficient and cost-effective biologics production. With regulators approving an increasing number of biologic therapies, the need for advanced bioreactor systems will continue accelerating.
Advances in single-use bioreactors enhancing market potential
One of the most transformative shifts in the bioreactors market is the widespread adoption of SUBs. Unlike traditional stainless-steel systems that require extensive sterilization and cleaning, SUBs use pre-sterilized disposable bags, eliminating cross-contamination risks and significantly reducing downtime between production cycles. This has made them particularly valuable for small-batch biologics, personalized therapies, and pandemic-driven rapid vaccine production.
A key advancement is the integration of continuous bioprocessing with single-use technologies, enabling uninterrupted manufacturing and higher throughput efficiency. Additionally, AI-powered bioprocess monitoring systems are revolutionizing SUB operations by providing real-time adjustments to parameters like pH, oxygen concentration, and glucose levels, ensuring optimal cell growth conditions. Another innovation is the emergence of modular and scalable SUB platforms, which allow manufacturers to expand production capacity without the constraints of fixed infrastructure.
Furthermore, regulatory bodies such as the FDA and EMA have increasingly endorsed single-use technologies, recognizing their role in reducing contamination risks and accelerating drug production timelines. As pharmaceutical companies continue seeking flexible and cost-efficient biomanufacturing solutions, SUBs will dominate the next phase of biologics production, driving innovation in reactor design, process control, and automation.
Competitive Landscape Analysis
The global bioreactors market is marked by the presence of established and emerging market players such as Thermo Fisher Scientific; Sartorius AG; GE Healthcare; Merck KGaA; Danaher Corporation; Eppendorf AG; Lonza Group AG; Solaris Biotechnology Srl.; Bioengineering AG; and Infors HT, among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
🔗 Want deeper insights? Download the sample report here:
https://meditechinsights.com/bioreactors-market/request-sample/
Global Bioreactors Market Segmentation
This report by Medi-Tech Insights provides the size of the global bioreactors market at the regional- and country-level from 2023 to 2030. The report further segments the market based on type, material, usage and end-user.
Market Size & Forecast (2023-2030), By Type, USD Million
- Single-Use Bioreactors
- Stirred-Tank Bioreactors
- Airlift Bioreactors
- Others
Market Size & Forecast (2023-2030), By Material, USD Million
- Glass
- Stainless Steel
- Single-Use
Market Size & Forecast (2023-2030), By Usage, USD Million
- Lab-Scale
- Pilot-Scale
- Large-Scale
Market Size & Forecast (2023-2030), By End-user, USD Million
- Biopharmaceutical Companies
- Academic & Research Institutes
- Contract Manufacturing Organizations (CMOs)
- Others
Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
info@meditechinsights.com