Decentralized clinical trials (DCT) employ a method of conducting clinical trials where parts or all of the trial happen outside a traditional physical clinic or trial site. Telemedicine, local/mobile healthcare providers, and digital/mobile technology are used to perform clinical trial studies.
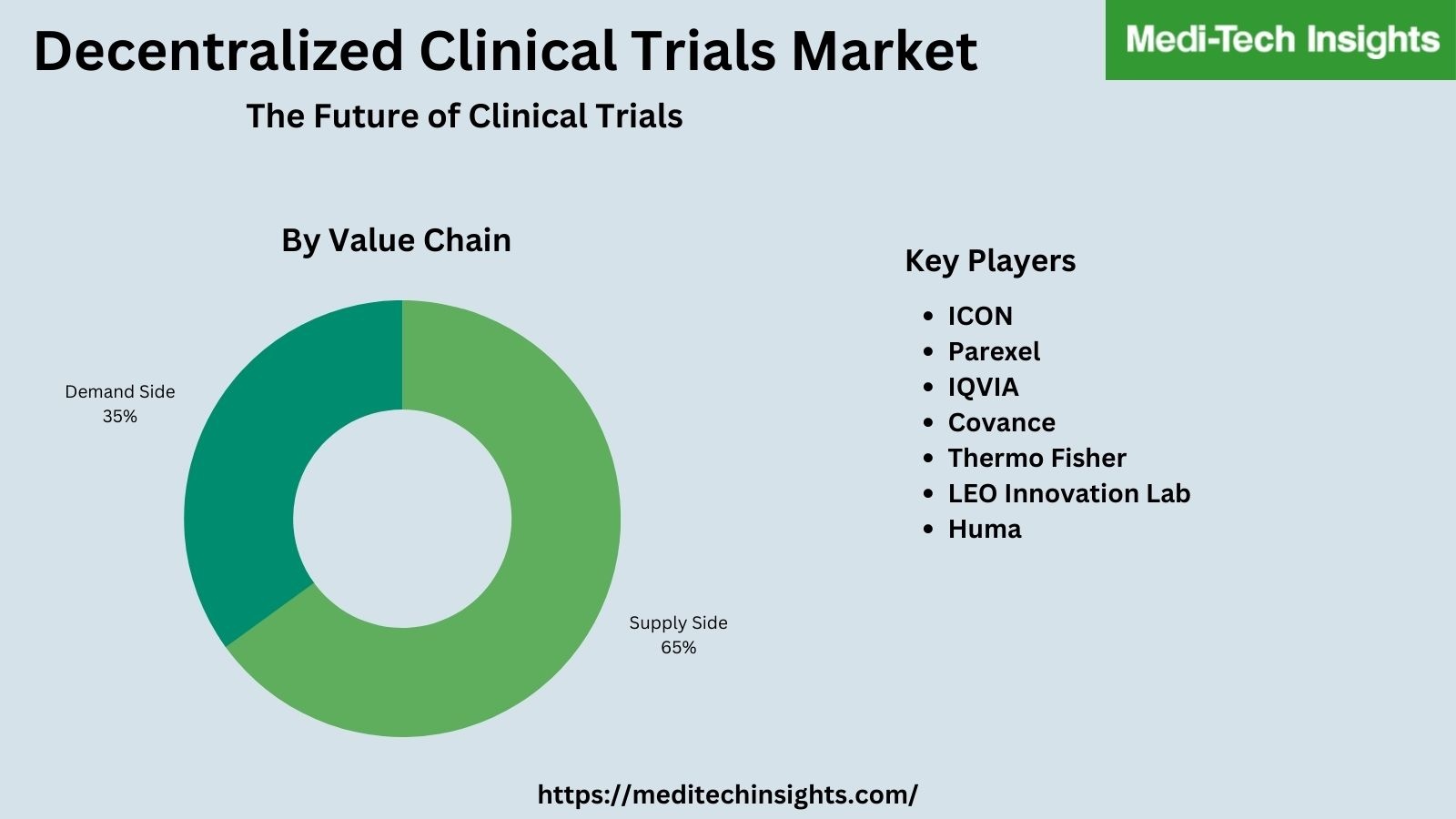
The Global Decentralized Clinical Trials (DCTs) Market valued at US$ 8.8 billion (2021) is set to witness a healthy growth rate of 10% to reach ~US$ 14.2 billion by 2026.
Some of the main factors propelling the global decentralized clinical trials (DCTs) market include the advantages of DCTs, Covid-19, growing adoption by pharmaceutical, medical device companies, and research organizations (CROs), formation of industry stakeholder groups like the Decentralized Trials & Research Alliance (DTRA) to facilitate collaboration and research, favourable funding & regulatory outlook, and surge in M&A activities. However, concerns around patient data privacy are likely to hamper the growth of the decentralized clinical trials (DCTs) market.
Explore Sample of Our Premium Report on Decentralized Clinical Trials (DCTs) Market @ https://meditechinsights.com/decentralized-clinical-trials-market/request-sample/
Covid-19 Spurs Adoption of Decentralized Clinical Trials (DCTs) Market
Internationally, Covid-19 had a negative influence on health care, and the clinical industry was not an exception. The challenges of doing clinical research during the Covid-19 pandemic resulted to the termination of more than 2000 trials that were registered on ClinicalTrials.gov. Covid-19 harmfully impacted participant recruitment, retention, the safety of trial subjects, protocol compliance, and highlighted the need for safe, reliable, and secure remote capabilities, which in turn led to a renewed focus on digitization.
Decentralized clinical trials (DCT) have become a vital tool in the wake of the Covid-19 pandemic, providing for the remote recruitment of patients, physician visits/patient consent can take place via telemedicine, and mobile technology can be used for remote data collection.
“The Covid-19 pandemic compelled several sponsors to incorporate virtual elements such as telemedicine, remote electronic medical record access for monitors, and virtual monitoring of data & study documentation into their trials.” – Senior Director, Leading DCT Solution Provider, United States