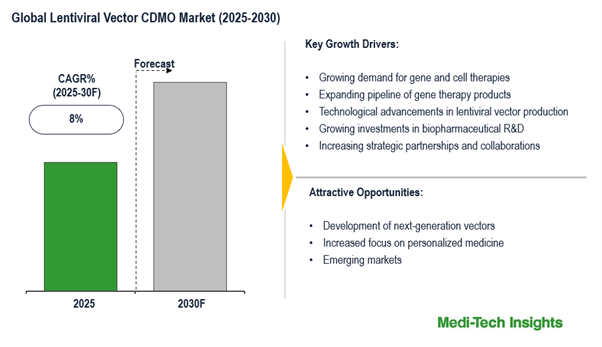
The global lentiviral vector CDMO market is set to witness a growth rate of 8% in the next 5 years. Growing demand for gene and cell therapies; expanding pipeline of gene therapy products; technological advancements in lentiviral vector production; growing investments in biopharmaceutical R&D and increasing strategic partnerships and collaborations are some of the key factors driving the lentiviral vector CDMO market.
Lentiviral vector contract development and manufacturing organization (CDMO) specializes in producing lentiviral vectors, critical for delivering genetic material in advanced therapies like CAR-T and gene therapies. These organizations offer comprehensive services, including vector design, process development, analytical testing, and large-scale GMP manufacturing. Leveraging state-of-the-art facilities and technical expertise, CDMOs ensure high-quality, regulatory-compliant production, helping biotech and pharmaceutical companies overcome manufacturing challenges. By partnering with CDMOs, companies can accelerate clinical trials, reduce time-to-market, and focus on research and innovation. Lentiviral Vector CDMOs play a vital role in advancing personalized medicine and addressing the growing demand for gene and cell therapies.
Unlock key findings! Fill out a quick inquiry to access a sample report
https://meditechinsights.com/global-lentiviral-vector-cdmo-market/request-sample/
Growing demand for gene and cell therapies to propel market demand
The growing demand for gene and cell therapies is a key driver of the lentiviral vector CDMO market growth. These therapies, such as CAR-T and gene editing, rely on lentiviral vectors to deliver genetic material into target cells. With an increasing approvals and clinical trials for gene and cell therapies, the need for scalable, high-quality vector manufacturing has surged. CDMOs offer advanced technologies, regulatory expertise, and large-scale production capabilities, enabling biotech and pharma companies to meet rising demands. This trend accelerates the development and commercialization of innovative therapies, solidifying the role of lentiviral vector CDMOs in the expanding biopharma industry.
Technological advancements in lentiviral vector production are driving the market growth
Technological advancements in lentiviral vector production are driving the lentiviral vector CDMO market by improving manufacturing efficiency, scalability, and cost-effectiveness. Innovations such as suspension-based production systems, optimized upstream and downstream processes, and automation enable higher yields and consistent quality. Advances in vector engineering, including self-inactivating and pseudotyped vectors, enhance safety and transduction efficiency, making them more suitable for clinical applications. These improvements attract biopharma companies seeking reliable, cutting-edge solutions for gene and cell therapies. By adopting these technologies, CDMOs can meet growing demand, reduce production timelines, and strengthen their position in the competitive biopharma landscape.
Competitive Landscape Analysis
The global lentiviral vector CDMO market is marked by the presence of established and emerging market players such as Thermo Fisher Scientific Inc., Catalent, Inc, ProBio, Revvity, Charles River Laboratories, Lonza, Oxford Biomedica PLC, Wuxi Advanced Therapies, ProBioGen AG, and AGC Biologics; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Get exclusive insights – download your sample report today
https://meditechinsights.com/global-lentiviral-vector-cdmo-market/request-sample/
Market Segmentation
This report by Medi-Tech Insights provides the size of the global lentiviral vector CDMO market at the regional- and country-level from 2023 to 2030. The report further segments the market based on phase, application, end user.
- Market Size & Forecast (2023-2030), By Phase, USD Million
- Preclinical
- Clinical
- Commercial
- Market Size & Forecast (2023-2030), By Application, USD Million
- Gene Therapy
- Cell Therapy
- Vaccines
- Others
- Market Size & Forecast (2023-2030), By End User, USD Million
- Pharmaceutical Companies
- Biotech Companies
- Other End Users
- Market Size & Forecast (2023-2030), By Region, USD Million
- North America
- US
- Canada
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
- North America
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79
info@meditechinsights.com
