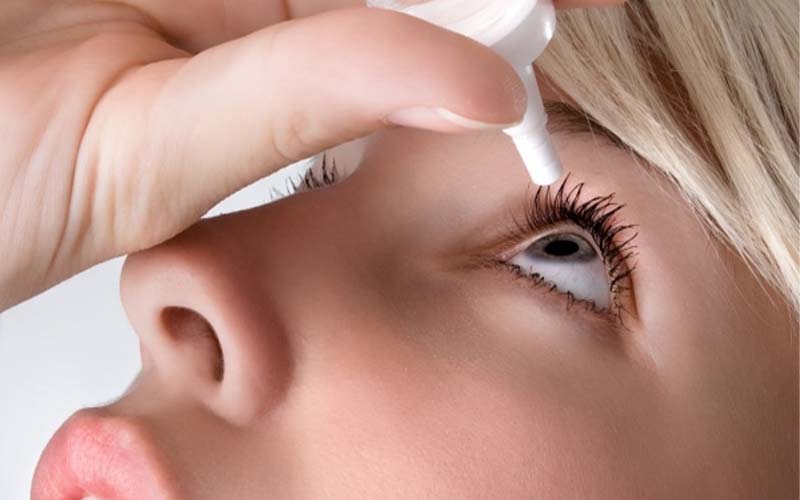
The majority of patients infected with Pseudomonas aeruginosa reported using artificial tears or eyedrops, according to the Centers for Disease Control and Prevention (CDC). This drug-resistant strain of bacteria caused severe injuries to 68 patients across the United States, including three deaths.
According to the CDC, these cases involved ten distinct ophthalmic drug brands. Ezricare Artificial Tears, which the Food and Drug Administration advised consumers to stop purchasing a month ago, were the most common.
The CDC stated that it will test unopened bottles to determine whether contamination occurred during manufacturing and that it confirmed a matching strain of Pseudomonas aeruginosa in the product’s opened bottles.
In February 2023, the FDA said that Ezricare’s parent company, Global Pharma Healthcare, a pharmaceutical provider based in India, had failed to provide adequate microbial testing for its over-the-counter eye product. The same was true for Delsam Pharma Artificial Eye Ointment, another of the company’s products, which the company voluntarily recalled shortly after.
According to the FDA, Global Pharma distributed the drugs without using appropriate preservatives and did not use adequate, tamper-evident packaging. NPR contacted Global Pharma for comment, but they did not immediately respond. In February 2023, eyedrop products from Apotex Corp. and Pharmedica USA were recalled, despite the fact that those brands’ products had not been linked to infections at the time.