The Growing Transplantation Market: Advancements, Challenges, and Strategies
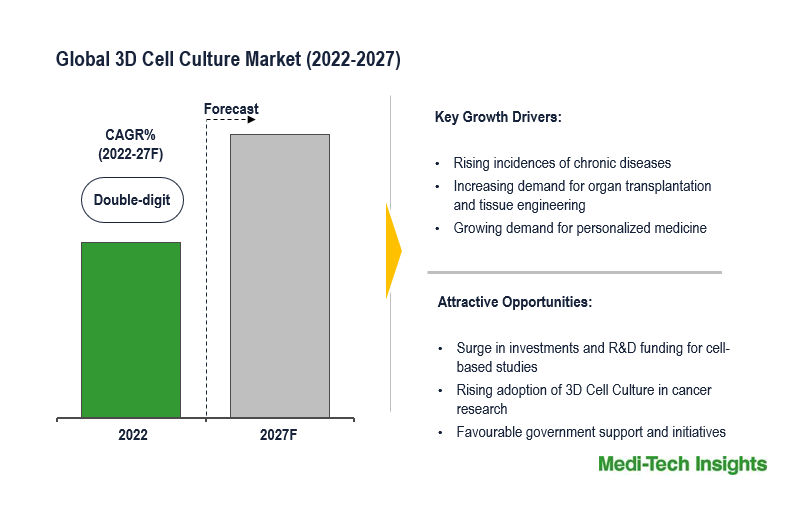
The transplantation market, encompassing organ and tissue transplantation, is poised for substantial growth between 2024 and 2029, with a projected Compound Annual Growth Rate (CAGR) of 9-10%. This surge is propelled by various factors such as technological advancements, increasing prevalence of chronic diseases leading to organ failure, rising awareness about transplantation, and evolving regulatory landscape. […]
Continue Reading